Understanding Lyme disease
What do The Platters and Lyme disease have in common? Both, although for totally different reasons, are associated with “The Great Pretender.” The Platters landed their “Great Pretender” connection through the 1955 recording of the song by the same name, which would go on to reach the #1 position on the R&B and pop charts just a year later.1 Lyme disease, on the other hand, is unfortunately connected to the term “The Great Pretender” because of its elusive, and often, non-specific nature of presentation.
Although Lyme disease (“Lyme”) was only recognized fairly recently – in the 1960s – its presence has been around for literally millennia. In fact, evidence of the bacterial DNA for Borrelia burgdorferi, the causative agent of Lyme disease, was found in a 5,300-year-old mummy dubbed “Ötzi the Iceman,” who was accidently discovered in 1991 by hikers in the Eastern Alps near the modern-day border of Austria and Italy.2,3
Yet, even after all of this time, many clinicians are still not up to speed on the potential, extensive havoc and debilitation this age-old pathogen can cause in the human body. Practitioners frequently lack experience in accurate diagnostic techniques and appropriate treatment options for their patients suffering from Lyme. Often patients may present with non-specific symptoms, including fatigue, pain, headache, and “feeling unwell”; these patients, if not diagnosed properly, may be given the spurious diagnosis of fibromyalgia or chronic fatigue syndrome, sending them into a spiral of treatments and recommendations that are ineffective, failing to address the infectious root cause of Lyme disease. Patient testimonials on Lyme abound on the web, as they feel marginalized, ostracized, depressed, and still sick, but with no answers. Lyme-literate, or at least Lyme-aware, practitioners are more crucial than ever, as Lyme disease cases are increasing across the nation.
This foundational review will hopefully make the clinical presentation, and therefore its diagnosis and treatment, less elusive. Several aspects of Lyme controversy will be explored, along with information on an appropriate and safe approach to provide care to Lyme patients in your practice. You will learn about early and late signs and symptoms, testing, diagnosis, testing, and treatment guidelines, including Functional Medicine approaches.

Background on Lyme disease
Many aspects of Lyme disease are surrounded by controversy and challenge, including:4
-
Accurate and timely diagnosis
-
Course of treatment
-
Acute- vs. post-treatment Lyme disease (PTLD), or chronic Lyme disease
-
Other controversies: Time of tick attachment (for transmission of disease vector), co-infections, health insurance concerns, and patient marginalization
Before attempting to discuss the details and implications of these frequently polarizing facets of Lyme, a greater understanding of the disease itself is necessary.
Lyme disease is the most common vector-borne disease in both the US and Europe.5,6 The Centers for Disease Control and Prevention (CDC) conservatively estimates that, in the US alone, more than 300,000 cases of Lyme disease are diagnosed annually.5 As seen in Figure 1, the geographical distribution of Lyme disease cases in the nation is concentrated in the Northeast and upper Midwest of the country; in fact, 14 states are estimated to account for over 95% of the cases reported to CDC.5
Figure 1: CDC. Reported Cases of Lyme Disease – United States, 2017.7

These particular geographical locations tend to produce the highest number of Lyme disease cases mainly due to their moist, undergrowth-rich environments, which is ideal for harboring and nurturing the blacklegged Ixodes ticks (see Figure 2), the offending vector for transmission of the infectious Borrelia species (often referred to as Borrelia burgdorferi sensu lato or Bb or Bb sensu lato). The blacklegged tick (sometimes called a deer tick), Ixodes scapularis (I. scapularis), inhabits the northeastern, mid-Atlantic, and north-central US, while the western blacklegged tick, Ixodes pacificus, resides on the Pacific Coast.8 In Europe the castor bean tick, Ixodes ricinus, is (one of) the culprit ticks, while in Asia it is the Ixodes persulcatus.9 The disease itself occurs across the US, Europe, the former Soviet Union region, and in China and Japan.9
The past two decades have seen a noteworthy up“tick” in the spread of I. scapularis in the US, mainly due to rapid environmental changes including land use variability (forest, agriculture, suburban, reforestation) and fluctuations in suitable animal host distribution.10 These changes have led to today’s extensive expansion of I. scapularis across the country, particularly in the northeast and mid-Atlantic states, in what researchers believe is “a species reclaiming its historical range.”10
Figure 2: Ixodes scapularis; Blacklegged Tick; Deer Tick.

Life cycle of Lyme disease ticks
The life cycle of the Ixodes tick is complex and requires a full 2 years to complete. Three essential criteria must be present for the full cycle to occur:8 1) Animals acting as a reservoir for Bb bacteria; 2) Ixodes ticks, which are infected and able to transmit the Bb bacteria; and 3) animal hosts to provide food for Ixodes ticks at the various stages of development (larva, nymph, adult). Figure 3 depicts the developmental stages for Ixodes ticks and transmission dynamics.
Figure 3: Developmental Stages of Ixodes Ticks and Dynamics of Transmission12

Garcia GR et al. 2016.12 https://creativecommons.org/licenses/by/4.0/. Accessed February 27, 2019.
Tick eggs are laid in spring and hatch into their larvae form in the summer. These larvae feed on rodents, small animals, and birds until early fall. If the animals which the ticks feed on happen to be reservoirs for the Bb bacteria, the tick may become infected and is now able to transmit this infection to other species, including humans. The following spring, the larvae transform into their nymph phase, and for the remainder of the summer and fall, will search for suitable sources of blood, typically small rodents, birds, and other small animals. It’s during this time frame that, if a human happens to wander into tick territory and is bitten by an infected tick, the Bb bacteria can inadvertently be transferred to the hapless human host.
In the fall, nymph ticks molt into adults, which prefer to feed on larger animals like deer. This will go on throughout the fall and into the next spring, at which time the adult female tick lays her next set of eggs, completing the life cycle of the tick.
Most commonly, humans are infected in spring and summer by nymph ticks, although they can also become infected if bitten by an adult tick in the fall or spring.11 Risk of infection to the inadvertent human host follows a bimodal age distribution pattern peaking at ages 5-15 and 45-59; the highest number of cases in the US is among boys aged 5-9 years old.11 In recent years, more infections have been reported in men than previously.11
Pathophysiology and Transmission of Lyme Disease

Garcia GR et al.2016.12 https://creativecommons.org/licenses/by/4.0/. Accessed February 27, 2019.
The transmission of Bb has been extensively studied to learn more about the factors necessary for successful infection of the mammalian host. This obligate parasite’s lack of true “virulence factors,” or common bacterial pathogenic factors such as lipopolysaccharides, toxins, and specialized secretion systems, indicate that the Bb species may not have evolved to cause disease in mammals; rather the mammals act only as reservoirs or inadvertent hosts, as is the case of the human host.14 In order to survive in the mammalian environment then, Bb possesses a set of specific characteristics; these can be subdivided into two groups:14
-
Factors involved in early infection such as outer-surface protein C (OspC) – these may be required for host colonization or resistance to innate immunity
-
Factors involved in resistance to acquired immunity such as the surface lipoprotein E known as variable major protein-like sequence expressed (VlsE)
Moreover, Bb exhibits bacterial factors including the alternative sigma factor pathway RpoN-RpoS, part of the signaling cascade that regulates gene expression for survival in varying environmental conditions.14 Further, it has been noted that Bb is notoriously adaptable expressing a wide array of survival mechanisms which, in addition to those described above, allow it to, alter its morphology, engage in antigenic variation, invoke periods of dormancy, inhabit protective niches, and form biofilms that protect the bacteria from the immune system and the effects of antibiotics.15
These survival tactics are responsible not only for supporting an infection in an unintentional mammalian host (such as a human),12 but per the International Lyme and Associated Diseases Society (ILADS), are also responsible for allowing Bb to persist in some individuals following a course of short-term antibiotic therapy, resulting in the controversial, chronic PTLD presentation, which will be discussed in more detail in a later section.4
Signs and Symptoms of Infection
The CDC details the clinical signs and symptoms observed in early and late detection of Lyme disease, as highlighted in Table 2:16
Table 2: Adapted from: CDC. Signs and Symptoms of Untreated Lyme Disease.16

Infection with Bb is often multi-systemic, typically involving the musculoskeletal, cardiovascular, and nervous systems. Early detection and treatment are key to recovery for most infected individuals, however, there is a subset of patients, who, despite early recommended antibiotic treatment, will unfortunately continue to exhibit chronic and debilitating symptoms, which ILADS has termed “chronic Lyme disease”4 or PTLD.
Clinical manifestation variations and virulence of the infection may be based on which Bb species is the offending agent. For example, B. afzelii infection is typically localized to skin manifestations, while B. garinii is usually associated with nervous system symptoms.17 Although B. burgdorferi is rarely seen in Europe, in the US it is associated with a greater number of disease-associated symptoms than are B. afzelii or B. garinii infections in Europe.17 The variations of symptoms seen across individuals is thought to be caused by the different host immune responses to each of the unique organisms, although all three aforementioned species of Borrelia typically elicit the classical erythema migrans (EM) lesion initially.17
Interestingly, B. burgdorferi infected patients show higher levels of mRNA for cytokines and chemokines associated with innate and Th1-adaptive immune responses in their erythema migrans lesions than do the patients infected with B. afzelii and B. burgdorferi.17 Specifically: “B. afzelii and B. burgdorferi–infected patients in the US also have higher levels of cytokines and chemokines in serum, and isolates from these patients induce macrophages to secrete more interleukin-6 (IL-6), IL-8, IL-10, chemokine ligand 3 (CCL3), CCL4, and tumor necrosis factor (TNF) than B. afzelii or B. garinii from patients in Slovenia.”17
Results show B. burgdorferi has a greater inflammatory potential than the other two strains, and the B. burgdorferi from the US appears to be more virulent than the B. burgdorferi from Europe; these unique reactions may also play a role in the clinical manifestation variations seen amongst Lyme patients.18
Diagnosis
The optimal method for accurate and timely diagnosis of Lyme disease has been a topic of heated debate since 1982, when Bb was first determined to be the causative agent for Lyme.3 Detecting the elusive spirochete, however, is easier said than done, as it only transiently enters the bloodstream of the infected individual and even then, only in small numbers.19 Indirect detection through antibody response of the patient has turned out to be a helpful method of assessment thus far, since direct detection approaches such a polymerase chain reaction (PCR) or culture present challenges.19
For example, PCR and culture have high sensitivity for skin samples of patients with an active EM and where diagnosis was made from clinical visualization of the lesion.20 However, PCR generally has low sensitivity on all extracutaneous sites in Lyme patients, except in synovial fluid where sensitivity is up to 90%; in this case, PCR could be useful as a confirmatory test but not as the primary diagnostic procedure.20
Two-tiered Testing for Lyme Disease
Indirect testing, such as in the 2-tiered serologic analysis (see Figure 5), involves an initial test consisting of a highly sensitive enzyme immunoassay (EIA) or immunofluorescence assay (IFA); if the result is positive or equivocal, it is followed by the highly specific Western immunoblot. If the patient’s symptoms have been present for < 30 days, then both the IgM and IgG Western blot are utilized, whereas if symptoms have been present for > 30 days, only the IgG Western blot is required.19
A negative first tier test, however, accompanied with signs/symptoms indicating possible Lyme infection, should be followed with further testing for alternative diagnosis or Lyme specific testing at a later date (after 30 days of symptom manifestation).21
Utilization of this 2-tiered testing produces a sensitivity of ~70%–100% and specificity > 95% for disseminated Lyme disease.19 This method of diagnosis has now become the “standard of care” in diagnosing Lyme disease, along with appropriate clinical judgement for ordering and interpreting the labs; 2-tiered testing is the only validated diagnostic approach for Lyme disease diagnosis recommended by CDC and the Public Health Agency of Canada.19,21
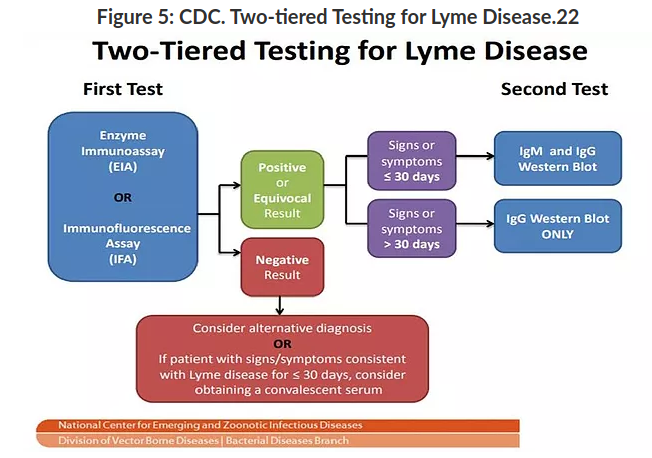
Both the first and second tier tests detect antibodies produced against Bb protein antigens. The first tier testing however has, until recently, used the whole-cell sonicate preparation of the spirochete as antigen for the EIA.19 This method, although creating high sensitivity because of multiple antigens in the whole cell preparation, can create false positive results due to the cross-reactivity potential between the infectious antigens and the host antigens or even with other pathogenic antigens present in the serum.19 For this reason, the specificity of the EIA without the second tier findings is not optimal for accurate Lyme diagnosis.19
Recently, the FDA has cleared EIAs with one-to-several specific Bb antigens, resulting in a higher specificity and similar sensitivity when compared to the whole-cell sonicate EIAs.19 Two such antigens being utilized in these newer tests include the cell surface VlsE lipoprotein and the C6 peptide.19 Both are highly conserved and immunogenic for pathogenic Lyme agents, thus creating an early antibody response; this approach may prove advantageous for Lyme diagnosis in the future.19
The second tier testing, Western immunoblot, is a serologic test that detects antibodies against a set of preselected Bb protein antigens rather than the whole-cell sonicate.19 The results from this test are time-dependent: positive IgM findings indicate an acute-phase, typically within 30 days of infection, whereas IgG-positive results may indicate a convalescent-phase, usually 30 days and beyond.19
Interpretation of the results can vary from practitioner to practitioner, presenting challenges in certain cases, particularly for false negative results. The recommendations for interpretation of the Western immunoblot are as follows:19
“A positive IgM Western immunoblot result is indicated by the scored presence of > 2 of 3 bands (21–24, 39, and 41 kDa), and a positive IgG result is indicated by the scored presence of > 5 of 10 bands (18, 21–24, 28, 30, 39, 41, 45, 58, 66, and 93 kDa). The 21–24-kDa band represents OspC, an outer surface protein with variable length and amino acid sequence.”
Further complicating the diagnostic field is the less-than-optimal detection of confirmed Lyme disease when the individual has been infected < 30 days, due in main part to the time required for the immune system of the human host to respond and mount a reaction.23 For this reason, the VlsE and C6 markers utilized in the first tier EIAs, as well as the OspC and FlaB marker from the feeding ticks, have proved to be potential “early warning system” indicators.23 Both the VlsE and C6 are conserved among Bb sensu lato genospecies and strains.23,24
Although diagnostic tests continue to evolve and improve, the emphasis on an appropriate and thorough physical exam and healthcare provider evaluation of the individual patient is imperative. Most obvious for the patient and healthcare provider will be the individual presenting with the hallmark EM rash/lesion; this presentation does not require additional serologic testing for definitive diagnosis.25 The EM is often referred to as the classic “bull’s eye” (see Figure 6) but can also present in various other forms:26
-
Expanding rash with central crust
-
Multiple rashes, disseminated infection
-
Red, oval plaque on trunk
-
Expanding rash with central clearing
-
Bluish rash, no central clearing
-
Red-blue lesion with central clearing
Figure 6: CDC. Classic, “Bull’s Eye” Lyme Disease Erythema migrans (EM) Rash.26

It is estimated that as many as 80-90% of patients will develop the EM after infection, usually within 3 to 30 days after the tick bite, but no later than 3 months.27,28 The EM is usually found on the lower extremities approximately 54% of the time and on the trunk approximately 29% of the time.28 The clinical bottom line for Lyme disease serology, considering EM lesion presence, is summarized below in Table 3.
Table 3: Clinical Bottom Line: Lyme Disease Serology. Adapted from: Lantos et al. 2016.29

As will be discussed further in the Treatment section of this review, an appropriate antibiotic prescription initiated during the acute phase (when EM is present) is reported to resolve the infection in 99% of patients.28 Unfortunately, there are individuals who either do not manifest, do not recognize, or do not seek treatment for the EM rash, in which case the infection spreads most typically to the skin, heart, central or peripheral nervous system, and joints.27,28
Although there has been a steady increase in Lyme awareness side by side with improved diagnostic measures, the number of Lyme disease cases is still estimated to be severely underreported. In fact, one group of researchers evaluating the results of testing for Lyme in seven large commercial laboratories estimated underreporting of Lyme cases to the CDC by 21.3- to 39.4-fold, while a second analysis by a different group of researchers looking at national health insurance claim coding and treatment regimens estimated an 11.5- to 14.6-fold underreporting of the disease.30,31
As can be seen, it is imperative that healthcare practitioners working with Lyme patients are able to:
-
Properly assess the signs and symptoms associated with Lyme disease presentation
-
Accurately interpret first and second tier testing results (if EM is not present)
-
Obtain an accurate and thorough Lyme-focused patient history
All three components will be important for an accurate and timely diagnosis and successful treatment.
Treatment for stages of lyme disease
Three well-accepted clinical stages of Lyme disease exist, each with varying manifestations and treatment recommendations. There is potentially a fourth, chronic stage which is heavily debated,32 which will be discussed in more detail below.
Stage 1
Early localized disease associated with EM and flu-like symptoms; Duration 1 to 30 days.
As previously mentioned, the EM skin rash may or may not resemble a bull’s eye. Non-specific symptoms may include chills, fever, fatigue, headache, stiff neck, muscle soreness, joint pain, swollen nodes, and/or sore throat. For individuals without a visible EM, or if EM is ignored or not treated, stage 1 symptoms alone may be easy to dismiss, thereby allowing the infection to progress to stage 2.32,33
Treatment Guidelines for Stage 1
The CDC’s current recommendation for early (3-30 days after tick bite), or stage 1 treatment, of Lyme are summarized in Table 4:16,34
Table 4: CDC. Lyme Disease Treatment.34

* Recent publications suggest the efficacy of shorter courses of treatment for early Lyme disease.34
As noted previously, this approach has been estimated to resolve up to 99% of acute Lyme cases if implemented within adequate time from initial infection; prior to the use of antibiotic treatment for Lyme disease, about 60% of untreated individuals developed Lyme arthritis, a symptom of late-stage Lyme disease.28,35
Resolution of symptoms typically occurs rapidly after antibiotic treatment, although some symptoms may take a few weeks to months to fully resolve.36 There is a low risk in approximately 10-15% of patients, who may experience a Jarisch-Herxheimer reaction, or worsening of symptoms due to die-off of the bacteria; if this occurs it will typically take place within the first 24 hours of starting antibiotic treatment and usually dissipates after 1-2 days.36
Stage 2
Early disseminated disease with malaise, pain, and flu-like symptoms; May affect the neurological, ocular, and musculoskeletal organs; Duration 3 to 10 weeks.
Early disseminated Lyme may occur several weeks or months after the tick bite as bacteria are beginning to spread throughout the body. In addition to the flu-like symptoms experienced in stage 1, this secondary stage is often characterized by increase in symptoms such as: chills, fever, headaches, fatigue, pain, weakness or
numbness in the extremities, changes in vision, cardiac symptoms such as palpitations or chest pain, rash, and/or Bell’s palsy.32,33
Treatment Guidelines for Stage 236
The current recommended treatments (see Table 5) for early disseminated Lyme infection include either oral or intravenous (IV) antibiotics, with the oral antibiotic approach used for cases that are considered less serious. This might include symptoms associated with skin lesions, nerve involvement (Bell’s or facial palsy) limited to cases not involving the brain or spinal cord, and mild cases of inflammation of the heart that may manifest as slight delays in conduction of electrical signals resulting in palpitations or chest pain. Some practitioners may recommend a spinal tap for cases involving nerves before making a recommendation for oral or IV antibiotics.
For the more serious symptoms such as Lyme meningitis or cardiovascular symptoms, which are considered more critical in nature, IV antibiotic therapy is typically utilized, commencing at the healthcare provider’s office or in the ER; follow up care and treatment are usually continued at home. It should be noted that in Europe, oral antibiotics seem to be just as effective as IV antibiotics for Lyme meningitis, and healthcare providers in the US are beginning to move more towards this approach.

Stage 3
Late disseminated Lyme disease chiefly affects the joints, muscles, and nerves; May last for months or years; Lyme arthritis is a hallmark symptom of this stage.
Progression to stage 3 Lyme disease occurs if the patient is not effectively treated in the first two stages; stage 3 symptoms may not present until weeks to years after the offending tick bite. Beyond the hallmark Lyme arthritis symptom of stage 3, which often presents in joints near the point of infection, there may also be an increase in neurological and cardiac symptoms experienced.31 Other symptoms may include: severe headaches and migraines, vertigo and dizziness, migrating pains in joints or tendons, stiff and aching neck, sleep disturbances and severe fatigue, continued heart rhythm problems, mental fogginess, processing and concentration issues, and numbness in extremities.33
Treatment Guidelines for Stage 3
Most conventional guidelines discuss additional IV antibiotic therapy if arthritis continues after initial oral antibiotic therapy (28-day course). However, in some cases, Lyme arthritis may persist even after the IV antibiotics, in which case other therapies such as hydroxychloroquine and/or synovectomy (surgical removal of the joint lining) are brought into the clinical conversation. Likewise, the stage 3-associated neurological conditions are also treated with IV antibiotics daily for 2-4 weeks.36
It has been noted that the late-disease manifestation of Lyme arthritis, which usually manifests months later, is accompanied by strong innate and adaptive immune responses with resulting production of antibodies.35 This increase in antibody production leads to the effective eradication of the bacterium when supported by oral and sometimes IV antibiotic therapy. 35
However, there is small group of individuals who do not see resolution of symptoms of Lyme arthritis after the antibiotic therapy, and a theory has evolved on the biological mechanism that perpetuates the condition.35 What is thought to occur when arthritic symptoms do not resolve, is that the highly inflammatory Bb strains, although fully eradicated, trigger high levels of synovial inflammation that resolve very slowly over time, not responding to additional antibiotic therapy – this has been termed “antibiotic-refractory arthritis” (ARA).35
Several crucial factors must be present to manifest ARA, including:35
-
Excessive inflammation during the infection
-
Infection-induced autoimmunity
-
Failure to downregulate inflammatory responses appropriately after killing off spirochetes
Moreover, specific HLA-DR alleles that bind an epitope of B. burgdorferi outer-surface protein A (OspA), leading to particularly strong Th1 responses, have been associated with ARA.38 Patients with the TLR1 single-nucleotide polymorphism (SNP) may have increased risk of ARA; the TLR1 SNP is associated with exceptionally high levels of cytokines and chemokines in affected joints, possibly leading to increased chances of unresolved and long-lasting inflammation even after the offending spirochete is eradicated.39 A published algorithm for treating ARA is as follows in Figure 7.
Figure 7: Algorithm for the Diagnosis and Treatment of Lyme Arthritis. Adapted from: Arvikar SL et al. 2015.35

Interestingly, there are few clinical guidelines detailing specific actions for any additional stage 3 symptoms that may persist after the recommended antibiotic approaches. In the case of ARA the literature suggests that time, along with the individual’s host immune system, will typically support the return to homeostasis, resulting in a disappearance of the arthritic symptoms, and that the use of disease-modifying anti-rheumatic drugs (DMARDS) may quicken this process.35
Unfortunately, in the past the medical community did not widely believe that symptoms other than the ARA could persist after antibiotic therapy; however, many patients felt and knew differently. Advocacy and non-conventional medical groups began to realize that there was a subset of patients who never fully recovered from their initial Lyme diagnosis, leading to potential long-term disability and patient marginalization. Over time, this group of patients was brought under the term of “post-treatment Lyme disease” or PTLD.40 Even now, some healthcare organizations do not acknowledge the existence of PTLD and its relationship to Lyme disease, although it appears the tide is slowly turning.
Post-treatment Lyme disease/syndrome (PTLD); occurrence is debatable.
The challenges surrounding appropriate Lyme treatment was neatly detailed in an article authored by Marcelo Campos, MD, a primary care doctor at Harvard Vanguard whose one of many interests includes the use of integrative and Functional Medicine. The article, published on the Harvard Health Blog, summarizes some key challenges surrounding Lyme treatment:
“….the controversy about the treatment is so intense that some have even coined the dispute ‘Lyme wars.’ The clash emerged from doctors’ offices, and spread to public hearings in statehouses around the country. One of the main points of contention is the duration of antibiotic treatment — not only for acute Lyme but also for PTLD. The evidence to recommend a specific length of antibiotics treatment is scarce.”41
Delving further into the controversy, it becomes clear that there are two competing schools of thought centering around whether PTLD (sometimes confused with the term “chronic Lyme disease”) actually exists and whether extended courses of antibiotics are effective against PTLD, and also what additional therapeutics may be helpful in reducing symptoms associated with PTLD.
At the time of this article, the 2006 guidelines from the Infectious Diseases Society of America (IDSA) entitled, “The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America”42 had been “archived” and an updated version was not available.43 The archived copy from these guidelines notes the following:42
“There is no convincing biologic evidence for the existence of symptomatic chronic B. burgdorferi infection among patients after receipt of recommended treatment regimens for Lyme disease. Antibiotic therapy has not proven to be useful and is not recommended for patients with chronic (> 6 months) subjective symptoms after recommended treatment regimens for Lyme disease…Therapeutic modalities not recommended. Because of a lack of biologic plausibility, lack of efficacy, absence of supporting data, or the potential for harm to the patient, the following are not recommended for treatment of patients with any manifestation of Lyme disease: first-generation cephalosporins, fluoroquinolones, carbapenems, vancomycin, metronidazole, tinidazole, amantadine, ketolides, isoniazid, trimethoprim-sulfamethoxazole, fluconazole, benzathine penicillin G, combinations of antimicrobials, pulsed-dosing (i.e., dosing on some days but not others), long-term antibiotic therapy, anti-Bartonella therapies, hyperbaric oxygen, ozone, fever therapy, intravenous immunoglobulin, cholestyramine, intravenous hydrogen peroxide, specific nutritional supplements, and others.”
Part of the frustration for the up to 20% of patients44 who may suffer the experience of PTLD is the high level of “non-belief” in symptom existence, in addition to the paucity of evidence-based “next-steps” to treat these symptoms. As can be imagined, this could quickly further the marginalization of patients from their family, friends, and providers when dealing with these “non-descript”, “non-diagnosable” set of symptoms.
To work on filling this palpable gap, ILADS published a review45 of data from 2 retrospective cohorts, 2 case series, a meta-analysis, 2 prospective European studies, and 4 NIH-sponsored clinical trials. The purpose of the review was to learn more about the long term consequences of Lyme disease.45 Some of the key findings from the review include:45
-
34% of a population-based, retrospective cohort were ill for an average of 6.2 years after antibiotic treatment.46
-
A meta-analysis of 504 patients treated for Lyme disease found that this patient group had more fatigue, musculoskeletal pain, and neurocognitive difficulties than 530 controls.47 Additionally, it demonstrated that persistent Lyme disease symptoms were a distinct set of symptoms, which differed from those of fibromyalgia, chronic fatigue syndrome, and depression.47
-
A European study of adults treated for neuroborreliosis found that at 30 months post-treatment, 16% were cognitively impaired.48
-
Using a combined total of 22 standardized measures of quality of life (QOL), fatigue, pain and cognition,49,-51 the investigators of the 4 NIH-sponsored retreatment trials documented that the Lyme patients’ QOL was consistently worse than that of control populations49-51 and equivalent to that of patients with congestive heart failure;51 pain levels were similar to those of post-surgical patients, and fatigue was on par with that seen in multiple sclerosis.49,51
In addition to the information distributed by ILADS, additional research is being conducted to learn more about the symptoms associated with PTLD, their etiology and, much needed safe and effective therapeutics.
Of particular interest is a 2018 preclinical study that found the presence of rare but persistent host-adapted spirochetes, residual antigen, and non-resolved inflammation in various tissues of doxycycline-treated rhesus macaques primates.52 These results support some of the earlier research indicating that PTLD may be, in part, triggered by unresolved inflammation resulting possibly, from the spirochetes initial infection and subsequent systemic dissemination.36,52 Further, these findings indicate that there may continue to be (albeit probably rare) host-adapted infecting organisms still present even after antibiotic treatment.52
Interesting new research has continued to add evidence that Bb is a highly adaptable organism with various mechanisms for evading the (animal) host immune system; these include its ability to:15
-
Exploit tick salivary proteins to delay early host immune responses
-
Deceive alternative complement pathways by masking surface antigens
-
Usurp the host’s plasminogen activating system
-
Continuously vary its surface antigens to frustrate humoral immune responses
-
Translocate using uniquely agile motility skills
-
Use chemotactic and niche-seeking traits to evade host immune traffic
-
Engage in quorum sensing and in biofilm-like behavior
-
Upgrade its genetic code through horizontal gene transfer (HGT)
-
Assume atypical morphologies that differ from its spirochetal form
-
Potentially form persister cells able to tolerate antibiotic challenge (thus far unproven)
It has been hypothesized that some or many of the above mechanisms may play a role in the persistent symptoms experienced by some individuals even post-antibiotic treatment. Further research continues to be pursued in this area.15
Functional and Integrative Medicine Approaches to Lyme Disease
Beyond the recommended guidelines for stage 1-3 Lyme disease, there is not a hard and fast, standardized integrative medicine protocol for Lyme patients. However, there are some commonly followed approaches that are often relied upon by Functional, integrative, and naturopathic physicians, and other healthcare providers who work with cases of Lyme disease. These recommendations treat the body from a holistic and systems biology approach. Through a detailed medical history and physical exam, paired with thorough diagnostic testing, the practitioner’s focus will most typically be on the following areas, individualized for the person presenting for treatment:53-55
-
Correct nutritional deficiencies
-
Repair damaged mucosal defenses
-
Address abnormal biofilms
-
Balance immune dysfunction; address underlying autoimmunity
-
Address co-infections
-
Reduce and resolve inflammation
-
Optimize mitochondrial function
-
Optimize hormonal balance
-
Improve overall health and lifestyle through individualized exercise, movement, sleep and stress modification recommendations
-
Assess for mental and emotional impact of chronic disease process on the individual and support accordingly
In summary, Lyme disease is a complex disease process with a potentially elusive diagnosis and debilitating trajectory when symptoms and clues are missed. Lyme disease research is still relatively new, so in time, more research will continue to elucidate the mechanisms for acute and post-treatment infections and their ensuing symptoms. These future investigations will further inform and validate successful and safe therapeutics.
What we do know however, is that Lyme disease, if recognized quickly, can be successfully treated in most cases with appropriate antibiotic therapy. Unfortunately, there is a subset of individuals who are either misdiagnosed, undiagnosed, or who do not respond favorably to the initial prescription of antibiotics. It is this group of patients who will continue to suffer and be marginalized if further research and successful therapeutics are not employed. Thankfully, with the emerging acceptance and work being done within various fields of medicine and the complementary use of integrative and Functional Medicine approaches, the promise of full resolution for complex, persistent cases is sure to be close.
This information is for educational purposes only. The statements have not been evaluated by the Food and Drug Administration and are not intended to diagnose, treat, cure, or prevent any disease. Consult your physician if you have any question regarding a medical condition.
-
Whitburn J. Top R&B/Hip-Hop Singles: 1942-2004. Billboard. 2004:463.
-
Kean WF et al. The musculoskeletal abnormalities of the Similaun Iceman (“ÖTZI”): clues to chronic pain and possible treatments. Inflammopharmacology. 2013;21(1):11–20.
-
Bay Area Lyme Foundation. History of Lyme disease. https://www.bayarealyme.org/about-lyme/history-lyme-disease/ Accessed February 28, 2019.
-
Controversies & challenges in treating Lyme and other tick-borne diseases. https://www.ilads.org/research-literature/controversies-challenges/ Accessed February 18, 2019.
-
How many people get Lyme disease? https://www.cdc.gov/lyme/stats/humancases.html Accessed February 18, 2019.
-
Vector-borne diseases. https://www.eea.europa.eu/data-and-maps/indicators/vector-borne-diseases-2/assessment Accessed February 23, 2019.
-
Reported cases of Lyme disease – United States, 2017. https://www.cdc.gov/lyme/datasurveillance/maps-recent.html Accessed February 26, 2019.
-
Lyme disease: What you need to know. https://www.cdc.gov/lyme/resources/brochure/lymediseasebrochure.pdf Accessed February 19, 2019.
-
Merck Manual. Lyme disease. https://www.merckmanuals.com/professional/infectious-diseases/spirochetes/lyme-disease Accessed February 19, 2019.
-
Eisen RJ et al. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 2018;34(4):295-309.
-
Schotthoefer AM et al. Ecology and epidemiology of Lyme borreliosis. Clin Lab Med. 2015;35(4):723-743.
-
Garcia GR et al. Lyme Disease: Vectors and Reservoirs. 2016. http://www.smgebooks.com/lyme-disease/chapters/LD-16-03.pdf. Accessed February 27, 2019.
-
Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370(18):1724-1731.
-
Tilly K et al. Biology of infection with Borrelia burgdorferi. Infect Dis Clin North Am. 2008;22(2):217-234.
-
Berndtson K. Review of evidence for immune evasion and persistent infection in Lyme disease. Int J Gen Med. 2013;6:291-306.
-
CDC. Signs and symptoms of untreated Lyme disease. https://www.cdc.gov/lyme/signs_symptoms/index.html Accessed February 21, 2019.
-
Cerar T et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis.2016;22(5):818-827.
-
Strle K et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis.2009;200(12):1936-1943.
-
Moore A et al. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis. 2016;22(7):1169–1177.
-
Aguero-Rosenfeld ME et al. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18(3):484-509.
-
Waddell LA et al. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One. 2016;11(12):e0168613.
-
CDC. Two-tiered testing for Lyme disease. https://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html February 28, 2019.
-
Embers ME et al. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin Vaccine Immunol. 2007;14(8):931–936.
-
Liang FT et al. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999;163(10):5566-5573.
-
Wright WF et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85(11):1086-1093.
-
CDC. Lyme disease rashes and look-alikes. https://www.cdc.gov/lyme/signs_symptoms/rashes.html Accessed February 21, 2019.
-
Lantos PM. Chronic Lyme disease: the controversies and the science. Expert Rev Anti Infect Ther. 2011;9(7):787-797.
-
Biesiada G et al. Lyme disease: review. Arch Med Sci. 2012;8(6):978–982.
-
Lantos PM et al. Lyme disease serology. JAMA. 2016;315(16):1780–1781.
-
Hinckley AF et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59(5):676–681.
-
Nelson CA et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis. 2015;21(9):1625–1631.
-
Skar GL et al. Lyme Disease. Treasure Island, FL. StatPearls Publishing. 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431066/. Accessed February 28, 2019.
-
Global Lyme Alliance. Stages. https://globallymealliance.org/about-lyme/diagnosis/stages/. Accessed February 23, 2019.
-
CDC. Treatment. https://www.cdc.gov/lyme/treatment/index.html. Accessed February 23, 2019.
-
Arvikar SL et al. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269–280.
-
Hu L et al. Patient education: Lyme disease treatment (beyond the basics). https://www.uptodate.com/contents/lyme-disease-treatment-beyond-the-basics Accessed February 23, 2019.
-
Sanchez E et al. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis. A review. JAMA. 2016;315(16):1767-1777.
-
Steere AC et al. Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clin Infect Dis. 2011;52(Suppl 3):S259–S265.
-
Strle K et al. Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64(5):1497–1507.
-
Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008;22(2):341–360.
-
Harvard Health Publishing. Lyme disease: resolving the “Lyme wars”. https://www.health.harvard.edu/blog/lyme-disease-resolving-the-lyme-wars-2018061814071. Accessed February 23, 2019.
-
Wormser GP et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9);1089–1134.
-
IDSA. Guidelines. https://www.idsociety.org/practice-guideline/alphabetical-guidelines/. Accessed February 23, 2019.
-
Lantos PM. Chronic Lyme disease. Infect Dis Clin North Am. 2015;29(2):325–340.
-
Cameron DJ et al. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014;12(9):1103-1135.
-
Shadick NA et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med. 1994;121(8):560-567.
-
Cairns V et al. Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol. 2005;34(6):1340-1345.
-
Eikeland R et al. European neuroborreliosis: quality of life 30 months after treatment. Acta Neurol Scand. 2011;124(5):349-354.
-
Fallon BA et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70(13):992-1003.
-
Krupp LB et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60(12):1923-1930.
-
Klempner MS et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345(2):85-92.
-
Crossland NA et al. Late disseminated Lyme disease: Associated pathology and spirochete persistence posttreatment in Rhesus macaques. Am J Pathol. 2018;188(3):672-682.
-
Hirani K. Lyme disease: the biomedical approach. https://www.metagenicsinstitute.com/maps/lyme-disease-the-biomedical-approach/. Accessed February 25, 2019.
-
Sult T. Assessment of chronic infections part 2: Lyme disease and other occult infections. Presented at Immune Advanced Practice Module, Institute for Functional Medicine, Atlanta GA. Presented on February 10, 2019.
-
Greenspan J. Rising Above Lyme Disease: A Revolutionary, Holistic Approach to Managing and Reversing the Symptoms of Lyme Disease and Reclaiming Your Life. Beverly, MA. Fair Winds Press. 2019.
Bianca Garilli, ND, USMC Veteran
Dr. Garilli is a former US Marine turned Naturopathic Doctor (ND). She works in private practice in Northern California and consults with naturopathic and Functional Medicine leaders, including the Institute for Functional Medicine and Metagenics. She is passionate about optimizing health and wellness in individuals, families, companies and communities- one lifestyle change at a time. Dr. Garilli has been on staff at the University of California Irvine, Susan Samueli Center for Integrative Medicine and is faculty at Hawthorn University. She is the creator of the Military and Veteran Health Initiative and is the current Past-President of the Children’s Heart Foundation, CA Chapter.


















